Cresol and Phenol are two terms that often confuse people due to their similar structures and properties. There are a few key contrasts between these two compounds that set them separated. We’ll investigate the dissimilarities between Cresol and Phenol, shedding light on their chemical compositions, employments, and impacts.
Definition of Cresol and Phenol
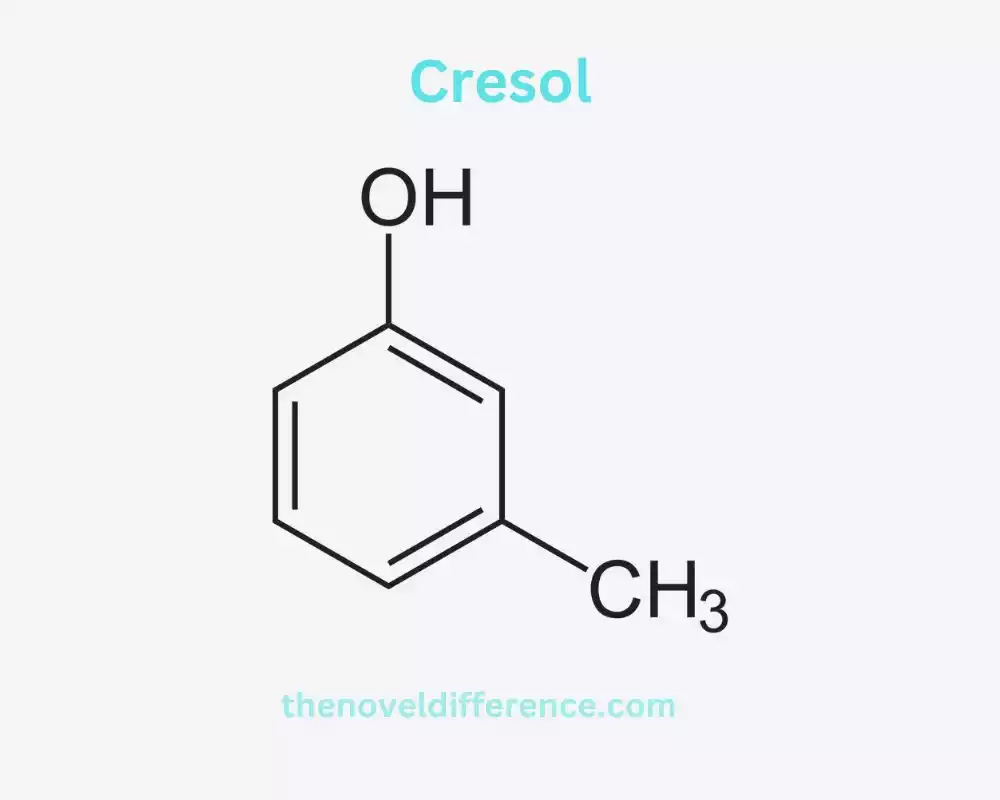
Definition of Cresol: Cresol alludes to a bunch of fragrant natural compounds that are determined from coal tar or petroleum. They are a sort of phenol, comprising a phenyl bunch (a fragrant ring) with a methyl gather (-CH3) connected to it. Cresols Can Exist in Completely Diverse Shapes, Tallying Ortho-Cresol (O-Cresol), Meta-Cresol (M-Cresol), and Para-Cresol (P-Cresol). They are colorless to pale yellow liquids with a distinct phenolic odor.
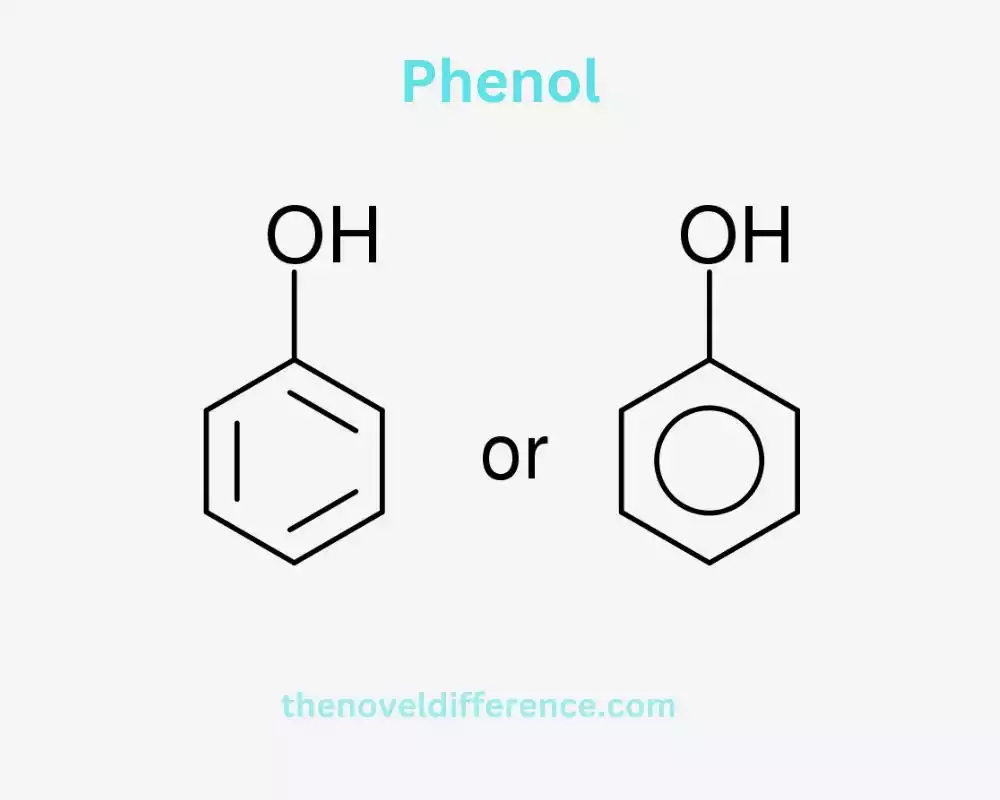
Definition of Phenol: Phenol, too known as carbolic corrosive, could be a straightforward organic compound and a fundamental building square within the chemistry of fragrant compounds. It comprises a phenyl bunch (a fragrant ring) with a hydroxyl bunch (-Gracious) joined to it. Phenol is a white crystalline solid that is volatile and has a characteristic sweet, medicinal odor. It is profoundly dissolvable in water and is commonly utilized as a beginning fabric for the generation of different chemicals, counting plastics, gums, and pharmaceuticals.
Importance and common uses of Cresol and Phenol
Importance and Common Uses of Cresol:
1. Disinfectants and Antiseptics: Cresols are widely used as active ingredients in disinfectants and antiseptic products. They have antimicrobial properties and are compelling against a wide extent of organisms, parasites, and contaminations.
2. Manufacturing of Resins and Plastics: Cresols are important in the production of resins, such as phenolic resins, which are widely used in various applications, including adhesives, coatings, and molded products.
3. Dyes and Pigments: Cresols are utilized in the manufacturing of dyes and pigments. They act as intermediates in the synthesis of colorants used in textiles, inks, and coatings.
Importance and Common Uses of Phenol:
1. Antiseptics and Preservatives: Phenol has strong antiseptic properties and is used in various antiseptic formulations, such as throat sprays and mouthwashes. It is additionally utilized as an additive in pharmaceuticals, beauty care products, and individual care items.
2. Plastics, Resins, and Adhesives: Phenol is a key raw material in the production of phenolic resins, which are widely used in the manufacturing of laminates, coatings, and molded products. It is also utilized in the synthesis of epoxy resins, which find applications in adhesives and coatings.
3. Pharmaceuticals and Pharmaceutical Intermediates: Phenol serves as an important starting material for the synthesis of various pharmaceutical compounds. It is utilized within the generation of analgesics, cleaning agents, and topical anesthetics. Phenol derivatives are also employed as intermediates in pharmaceutical synthesis.
Both cresol and phenol are significant in various industries due to their antimicrobial properties, chemical reactivity, and utility as raw materials. It is critical to note that these compounds ought to be taken care of and utilized with caution due to their harmfulness and potential well-being dangers.
What is Cresol?
Cresol, too known as methyl phenol, is a natural compound comprising a phenol particle with a methyl bunch (-CH3) connected to it. It Can Exist in Three Isomeric Shapes: Ortho-Cresol (O-Cresol), Meta-Cresol (M-Cresol), and Para-Cresol (P-Cresol). These isomers differ in the position of the methyl group relative to the hydroxyl group (-OH) on the benzene ring.
Uses of Cresol
Cresol finds applications in various industries due to its antimicrobial and disinfectant properties. It is commonly utilized as a forerunner within the generation of gums, pesticides, and solvents. Moreover, cresol is a basic fixing within the fabricating of manufactured scents, colors, and pharmaceuticals.
Health Effects and Precautions
Exposure to cresol can have adverse health effects. Inhalation or direct contact with cresol can cause skin irritation, eye damage, respiratory issues, and gastrointestinal problems. Prolonged exposure may even lead to liver and kidney damage. Hence, it is crucial to handle cresol with care and follow safety guidelines when using it.
What is Phenol?
Phenol, moreover known as carbolic corrosive, could be a cyclic fragrant compound with the equation C6H6O. It comprises a benzene ring with a hydroxyl bunch (-Goodness) connected to it. The chemical structure of phenol makes it highly reactive and capable of forming various derivatives.
Uses of Phenol
Phenol has numerous industrial applications, thanks to its unique properties. It is broadly utilized as a crude fabric within the generation of plastics, tars, and engineered strands. In addition, phenol plays an imperative part in the manufacturing of pharmaceuticals, cleaning agents, and disinfectants. It is additionally utilized in restorative items, such as moisturizers and creams.
Health Effects and Precautions
Phenol can be toxic and corrosive, posing health risks if not handled properly. Presentation of tall concentrations of phenol can cause skin burns, respiratory issues, and eye harm. It is basic to require essential safeguards, such as wearing defensive clothing and utilizing appropriate ventilation when working with phenol.
Chemical Structure and Properties
Chemical Structure and Properties of Cresol: Cresol may be a phenolic compound with the chemical equation C7H8O. It consists of a phenyl group (C6H5), which is an aromatic ring, and a methyl group (-CH3) attached to it. The position of the methyl group can vary, resulting in different isomers of cresol.
The three isomers of cresol are:
1. Ortho-Cresol (o-Cresol): The methyl group is attached to the ortho position (adjacent carbon atoms) of the aromatic ring.
2. Meta-Cresol (m-Cresol): The methyl group is attached to the meta position (alternate carbon atoms) of the aromatic ring.
3. Para-Cresol (p-Cresol): The methyl group is attached to the para position (opposite carbon atoms) of the aromatic ring.
Cresols are aromatic compounds, meaning they possess an aromatic ring that consists of alternating double and single bonds. The presence of the methyl group on the ring influences the chemical and physical properties of cresol.
Physical Properties of Cresol:
1. State: Cresols are typically colorless to pale yellow liquids at room temperature.
2. Odor: Cresols have a distinct phenolic odor, which is often described as medicinal or disinfectant-like.
3. Melting Point: The melting points of cresols vary depending on the isomer. Ortho-cresol encompasses a dissolving point of around 31°C, meta-cresol softens at around 10°C, and para-cresol features a higher softening point of around 35°C.
4. Boiling Point: The boiling points of cresols also differ among the isomers. Ortho-cresol contains a bubbling point of around 191°C, meta-cresol bubbles at roughly 202°C, and para-cresol contains a higher bubbling point of around 202-203°C.
5. Solubility: Cresols are soluble in organic solvents such as ethanol, ether, and chloroform. However, their solubility in water is limited.
The specific chemical and physical properties of cresol may vary slightly depending on the isomer and the presence of other functional groups if any.
Physical properties of Cresol
Physical properties of Cresol:
1. State: Cresols are typically colorless to pale yellow liquids at room temperature. They exist as liquid compounds.
2. Odor: Cresols have a distinct phenolic odor, which is often described as medicinal or disinfectant-like. The odor can be very solid and sharp.
3. Melting Point: The melting points of cresols vary depending on the isomer. For ortho-cresol, the melting point is around 31°C. Meta-cresol has a lower melting point of approximately 10°C. Para-cresol has a higher melting point of around 35°C.
4. Boiling Point: Cresols have relatively high boiling points. Ortho-cresol has a boiling point of around 191°C. Meta-cresol and para-cresol have similar boiling points, both around 202-203°C.
5. Density: The density of cresols ranges from approximately 1.03 g/cm³ to 1.04 g/cm³.
6. Solubility: Cresols are soluble in organic solvents such as ethanol, ether, and chloroform. However, their solubility in water is limited. They are considered partially soluble in water, forming a turbid or milky solution due to their hydrophobic nature.
It is critical to note that these physical properties can change somewhat depending on components such as pollution, temperature, and a particular isomer of cresol.
Melting point
The melting points of cresols vary depending on the specific isomer.
Here are the approximate melting points for each isomer of cresol:
1. Ortho-Cresol (o-Cresol): The melting point of ortho-cresol is around 31°C.
2. Meta-Cresol (m-Cresol): Meta-cresol has a lower melting point compared to the other isomers, with an approximate melting point of about 10°C.
3. Para-Cresol (p-Cresol): The melting point of para-cresol is higher compared to the other isomers, with an approximate melting point of around 35°C.
It’s vital to note that these values are inexact and can change depending on components such as immaculateness, test conditions, and particular gem shapes.
Boiling point
The boiling points of cresols also vary depending on the specific isomer.
Here are the approximate boiling points for each isomer of cresol:
1. Ortho-Cresol (o-Cresol): The boiling point of ortho-cresol is around 191°C.
2. Meta-Cresol (m-Cresol): Meta-cresol has a slightly higher boiling point compared to ortho-cresol, with an approximate boiling point of around 202°C.
3. Para-Cresol (p-Cresol): The Boiling Point of Para-Cresol Is Similar to That of Meta-Cresol, With an Approximate Boiling Point of Around 202-203°C.
It’s critical to note that these values are surmised and can be affected by variables such as immaculateness, barometrical weight, and particular exploratory conditions.
Comparison Chart
Here is a comparison chart highlighting the key differences between cresol and phenol:
| Properties | Phenol | Cresol |
|---|---|---|
| Chemical Formula | C6H6O | C7H8O |
| Structure | Hydroxyl (-OH) group attached to a benzene ring | Hydroxyl (-OH) group and an additional methyl (-CH3) group attached to a benzene ring |
| Isomers | N/A | Ortho-cresol (o-cresol), meta-cresol (m-cresol), para-cresol (p-cresol) |
| Melting Point | Approximately 40-42°C | Varies: o-cresol ~ 31°C, m-cresol ~ 10°C, p-cresol ~ 35°C |
| Boiling Point | Approximately 181-187°C | Varies: o-cresol ~ 191°C, m-cresol and p-cresol ~ 202-203°C |
| Acidity | Stronger acid | Weaker acid compared to phenol |
| Reactivity | Similar to cresol | Similar to phenol with some variations due to the presence of the methyl group |
| Applications | Resins, plastics, pharmaceuticals, disinfectants | Resins, disinfectants, pharmaceuticals, solvents, intermediates |
Uses and Applications
Phenol has a wide range of uses and applications across various industries.
Some of the common uses and applications of phenol include:
1. Plastics and Resins: Phenol is a key ingredient in the production of phenolic resins. These gums are broadly utilized within the fabricating of molded items, coatings, cement, and covers. Phenolic resins have excellent heat resistance, mechanical strength, and electrical insulation properties.
2. Pharmaceuticals: Phenol serves as a starting material for the synthesis of various pharmaceutical compounds. It is utilized within the generation of analgesics, cleaning agents, disinfectants, and topical anesthetics. Phenol derivatives are also employed as intermediates in pharmaceutical synthesis.
3. Personal Care Products: Phenol and its derivatives are used in the formulation of certain personal care and cosmetic products. They are utilized in mouthwashes, throat showers, skin salves, and haircare items for their clean properties.
4. Agricultural Chemicals: Phenol is sometimes used in agricultural applications as a wood preservative to protect against decay and insect damage. It has moreover been utilized as a herbicide, even though its utilization in this zone is constrained due to natural concerns.
5. Industrial Chemicals: Phenol is a versatile chemical that finds applications in the production of various industrial chemicals. It is utilized as an antecedent for the union of materials like bisphenol A, caprolactam (utilized in nylon generation), and alkylphenols (utilized in surfactants and cleaners).
6. Adhesives and Sealants: Phenolic resins derived from phenol are utilized in the formulation of adhesives and sealants due to their excellent bonding properties and resistance to heat and chemicals.
7. Laboratory and Research: Phenol is commonly used in laboratories as a reagent and solvent for various chemical reactions and extractions. It is additionally utilized in atomic science methods, such as DNA and RNA extraction.
It is imperative to note that phenol can be harmful and ought to be dealt with with caution. Proper safety precautions and guidelines must be followed when working with phenol-containing substances to ensure safe handling and minimize exposure.
Disinfectants and antiseptics
Phenol is broadly utilized as a dynamic fixing in disinfectants and cleaning agents due to its solid antimicrobial properties.
Here’s how it is utilized in these applications:
Disinfectants: Phenol-based disinfectants are commonly used to kill or inhibit the growth of microorganisms on surfaces. They are compelling against a wide extent of microscopic organisms, parasites, and infections. Phenol-based disinfectants are utilized in different settings, counting healthcare offices, research facilities, and family units, to clean surfaces, hardware, and disobedient. These disinfectants are especially valuable in high-risk regions where the control of irresistible specialists is significant.
Antiseptics: Phenol is additionally utilized as a dynamic fixing in clean items. Cleaning agents are substances that are connected to living tissues, such as the skin, to decrease the hazard of contamination. Phenol-based cleaning agents are utilized for cleaning and cleansing wounds, cuts, and scraped areas. They offer assistance anticipate the development of microorganisms and diminish the chance of auxiliary contaminations.
Phenol acts by disrupting the cell membranes and denaturing proteins of microorganisms, leading to their destruction or inhibition of growth. Its broad-spectrum antimicrobial movement makes it successful against a wide run of pathogens, counting microscopic organisms, infections, and organisms.
It’s vital to note that whereas phenol is viable as a disinfectant and clean, it can be harmful on the off chance that utilized despicably or in tall concentrations. Phenol ought to be utilized concurring to informational, and safeguards ought to be taken to dodge coordinate contact with the skin and eyes.
Manufacturing of resins and plastics
Phenol plays a crucial role in the manufacturing of resins and plastics, particularly phenolic resins.
Here’s how phenol is utilized within the generation of gums and plastics:
Phenolic Resins: Phenolic gums, too known as phenol-formaldehyde gums, are a sort of thermosetting gum broadly utilized in different businesses. They are formed by the condensation polymerization of phenol with formaldehyde in the presence of a catalyst. Phenol serves as the primary monomer in the synthesis of these resins.
The manufacturing process typically involves the following steps:
1. Condensation Reaction: Phenol is mixed with formaldehyde and a catalyst, usually an acid or base. The reaction between phenol and formaldehyde results in the formation of novolac or resol-type phenolic resins, depending on the molar ratio of the reactants and the reaction conditions.
2. Crosslinking: The novolac or resol phenolic resin is then cured or crosslinked using heat and a curing agent. This process creates strong and durable three-dimensional networks, leading to the hardening and solidification of the resin.
3. Formulation: Depending on the desired application, other additives, fillers, and reinforcements may be incorporated into the resin formulation to enhance specific properties, such as fire resistance, heat resistance, or mechanical strength.
4. Molding and Curing: The resin mixture is then molded into the desired shape and subjected to heat and pressure to complete the curing process. This step ensures the final product retains its shape and acquires the desired mechanical and chemical properties.
Phenolic resins have excellent heat resistance, high mechanical strength, good electrical insulation properties, and chemical resistance. They find extensive use in a wide range of applications, including adhesives, coatings, molded products, laminates, insulation materials, and friction materials.
Other Plastics: Phenol is additionally utilized within the blend of other plastics, counting polycarbonates, epoxy tars, and polyester gums. Phenol serves as a building block or raw material that undergoes polymerization reactions with other monomers or chemicals to form the final plastic product.
P phenol is a vital component in the manufacturing of phenolic resins and is involved in the production of various other plastics. These resins and plastics find wide application across industries due to their desirable properties and versatility.
Conclusion
While Cresol and Phenol may share some similarities, they are distinct compounds with different properties and applications. Cresol exists in various isomeric forms, whereas phenol does not exhibit isomerism. Both compounds discover utility in numerous businesses, such as pharmaceuticals, plastics, and scents, but with shifting degrees of harmfulness. Understanding the dissimilarities between cresol and phenol is crucial for safe handling and informed decision-making in their respective applications.
