Gay Lussac’s Law and Avogandro’s Law what is the difference between them? Today, In this article you are going to learn about Gay Lussac’s Law and Avogadro’s Law. So, let’s get started.
Importance of Gay Lussac’s Law and Avogadro’s Law
The importance of Gay-Lussac’s Law and Avogadro’s Law lies in their fundamental contributions to our understanding of the behavior of gases.
These laws provide valuable insights into the relationships between different properties of gases and have numerous applications in various scientific and practical fields. Here is the specific importance of each law:
Gay Lussac’s Law:
- Predicting and Understanding Gas Behavior: Gay-Lussac’s Law establishes a direct relationship between the pressure and temperature of a gas at constant volume. This law allows scientists and engineers to predict the effect of changing temperature on the pressure of a gas, and vice versa. It helps in understanding how gases behave under different conditions, such as in the compression of gases, gas expansion, and gas reactions.
- Ideal Gas Law: Gay-Lussac’s Law, along with other gas laws, contributes to the development of the ideal gas law. The ideal gas law combines the relationships between pressure, volume, temperature, and the number of gas molecules to describe the behavior of ideal gases. It is widely used in various scientific calculations, including in chemistry, physics, and engineering.
- Industrial Applications: Gay-Lussac’s Law finds applications in various industrial processes. Understanding the relationship between temperature and pressure in LPG production is integral for its safe storage and transport. It also has implications in gas storage, gas pressure regulation, and control systems.
Avogadro’s Law:
- Molar Volume and Stoichiometry: Avogadro’s Law establishes a direct relationship between the volume of a gas and the number of gas molecules (moles) at constant temperature and pressure. This law enables scientists to determine the molar volume of gases and use it for stoichiometric calculations. It provides a link between the macroscopic properties of gases, such as volume, and the microscopic level of individual gas molecules.
- Standard Conditions: Avogadro’s Law is crucial for defining the standard conditions used in scientific measurements and calculations. The concept of the Avogadro constant (6.022 x 10^23), derived from Avogadro’s Law, allows scientists to relate the number of gas molecules to measurable quantities, such as volume and mass. Standard conditions (0 degrees Celsius, 1 atmosphere pressure) are used as a reference for comparing gas properties and performing standardized experiments.
- Gas Density and Gas Analysis: Avogadro’s Law plays a significant role in determining the density of gases. By knowing the volume occupied by a specific number of gas molecules, one can calculate the density of the gas. This information is valuable in gas analysis, including determining the composition of gas mixtures, such as in air quality monitoring and gas chromatography.
Gay-Lussac’s Law and Avogadro’s Law are both essential in understanding and predicting the behavior of gases. They provide valuable insights into the relationships between different gas properties and have wide-ranging applications in various scientific, industrial, and practical fields.
A brief explanation of the gas laws
The gas laws are a set of fundamental principles that describe the behavior of gases under different conditions. These laws establish relationships between the physical properties of gases, such as pressure, volume, temperature, and the number of gas molecules.
Understanding and applying the gas laws are crucial in various scientific disciplines, including chemistry, physics, and engineering. Here is a brief explanation of the three most common gas laws:
- Boyle’s Law: Boyle’s Law states that, at a constant temperature, the pressure of a gas is inversely proportional to its volume. Simply stated, as volume decreases and pressure rises simultaneously (assuming temperature remains constant), volumetric change causes pressure changes as volume shrinks; conversely vice versa. Mathematically, Boyle’s Law can be represented as P1V1 = P2V2, where pressure (P) represents pressure while volume (V) represents volume.
- Charles’s Law: Charles’s Law states that, at a constant pressure, the volume of a gas is directly proportional to its temperature measured on an absolute scale (usually Kelvin). As temperature changes affect gas volumes, expansion increases while reduction decreases as long as pressure remains constant. Charles’ Law can be expressed mathematically as V1/T1 = V2/T2, where V denotes volume and T temperature.
- Gay-Lussac’s Law: Gay-Lussac’s Law, also known as the Pressure-Temperature Law, states that, at a constant volume, the pressure of a gas is directly proportional to its temperature measured on an absolute scale. This law establishes a relationship between pressure and temperature of gasses, showing how as the temperature of a gas increases so does its pressure; conversely as its temperature declines so does its pressure, while their volumes remain consistent. Mathematically, Gay-Lussac’s Law is expressed as P₁/T₁ = P₂/T₂, where P represents pressure and T represents temperature.
These gas laws, along with Avogadro’s Law and the ideal gas law, form the foundation for understanding the behavior of gases.
They provide valuable insights into the relationships between gas properties and are essential in various scientific calculations, gas behavior predictions, and practical applications.
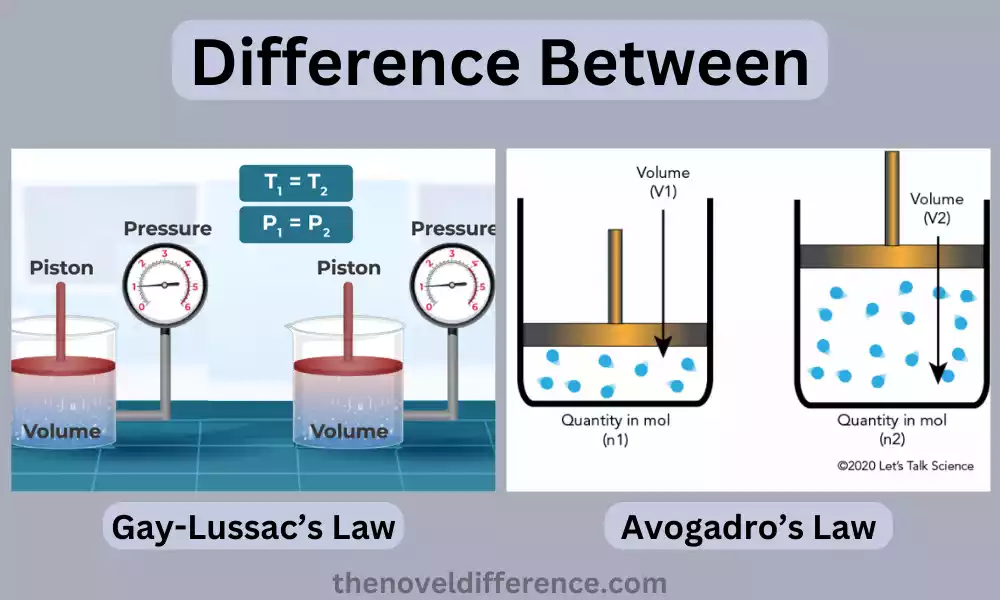
Gay Lussac’s Law
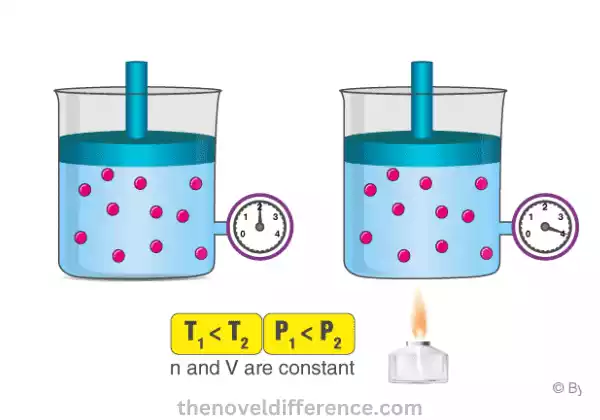
Gay-Lussac’s Law, also known as the Pressure-Temperature Law, describes the relationship between the pressure and temperature of a gas at a constant volume.
It is named after the French chemist Joseph Louis Gay-Lussac, who formulated the law in the early 19th century based on his experimental observations.
The statement of Gay-Lussac’s Law can be summarized as follows: “The pressure of a given amount of gas is directly proportional to its temperature, provided the volume remains constant.”
Mathematically, Gay Lussac’s Law can be expressed as:
P₁/T₁ = P₂/T₂
Where P1 and P2 represent the initial and final pressures, respectively; T1 and T2 refer to initial and final temperatures respectively of the gas.
Key points about Gay-Lussac’s Law:
- Direct Relationship: According to Gay-Lussac’s Law, as the temperature of a gas increases, its pressure also increases, assuming the volume remains constant. Similarly, if the temperature decreases, the pressure of the gas decreases, again with the condition of constant volume.
- Constant Volume: Gay-Lussac’s Law applies when the volume of the gas remains unchanged throughout the process being observed. If the volume changes, the law no longer holds, and other gas laws, such as Boyle’s Law or Charles’s Law, come into play.
- Experimental Evidence: Gay-Lussac’s Law is based on experimental observations, particularly those involving gases at high temperatures. Through his experiments, Gay-Lussac found that the pressure of a gas increased in direct proportion to the temperature increase, provided the volume was constant.
- Real-World Applications: Gay-Lussac’s Law has significant practical applications. It is crucial in various industries, such as gas storage and transportation, where understanding the relationship between pressure and temperature is important for maintaining safe conditions. It also finds application in gas pressure regulation, control systems, and other areas where gases are involved.
- Combined Gas Law: Gay-Lussac’s Law is one component of the combined gas law, which incorporates Boyle’s Law and Charles’s Law. The combined gas law allows for the calculation of changes in pressure, volume, and temperature simultaneously, given initial and final conditions of a gas sample.
Gay-Lussac’s Law describes the direct relationship between the pressure and temperature of a gas at a constant volume. It is a fundamental principle in understanding gas behavior and has practical applications in various industries.
Definition and statement of the law
Gay-Lussac’s Law, also known as the Pressure-Temperature Law, states that the pressure of a given amount of gas is directly proportional to its temperature, provided the volume remains constant.
Mathematically, the law can be expressed as:
P₁/T₁ = P₂/T₂
Where:
- P₁ and P₂ represent the initial and final pressures of the gas, respectively.
- T₁ and T₂ represent the initial and final temperatures of the gas, respectively.
Gay-Lussac’s Law states, in essence, that when holding volume constant for any given gas, increasing temperature causes its pressure to increase while decreasing it decreases it.
This law applies to ideal gases and is valid when the volume of the gas does not change during the process under consideration. It is an empirical law derived from experimental observations and has found wide applications in various fields, such as chemistry, physics, engineering, and industry.
Gay-Lussac’s Law is essential for understanding and predicting the behavior of gases under different conditions. It helps in analyzing gas reactions, determining the effect of temperature changes on gas pressure, and ensuring the safe storage and handling of gases in various applications.
Avogadro’s Law
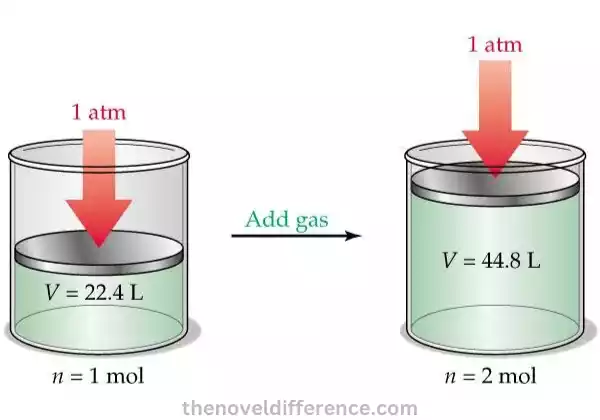
Avogadro’s Law, also known as the Law of Volumes, states that equal volumes of gases, at the same temperature and pressure, contain an equal number of molecules, regardless of their chemical nature or molar mass.
The statement of Avogadro’s Law can be summarized as follows: “Equal volumes of gases, under the same conditions of temperature and pressure, contain an equal number of molecules.”
Key points about Avogadro’s Law:
- Equal Volumes: Avogadro’s Law focuses on the relationship between the volume of a gas and the number of gas molecules. According to this theory, when two gases with identical temperatures and pressure conditions exist in equal volumes, their volumes will directly correspond with their molecular counts.
- Constant Temperature and Pressure: Avogadro’s Law holds true when the gases being compared are at the same temperature and pressure. This means that the law is applicable in ideal gas conditions, where temperature and pressure remain constant.
- Equal Number of Molecules: Avogadro’s Law implies that gases have the same number of molecules per unit volume at the same temperature and pressure. This is irrespective of the chemical nature, molar mass, or other properties of the gases.
- Molar Volume: Avogadro’s Law allows for the determination of the molar volume of gases. The molar volume represents the volume occupied by one mole of a gas at a specific temperature and pressure. Under standard conditions (0 degrees Celsius and 1-atmosphere pressure), the molar volume is approximately 22.4 liters.
- Stoichiometry: Avogadro’s Law is essential in stoichiometric calculations, as it provides a connection between the macroscopic volume of gases and the number of gas molecules. It allows for the establishment of mole-to-volume relationships in chemical reactions.
- Gas Density: Avogadro’s Law can be used to compare the densities of different gases under the same conditions. Since equal volumes contain an equal number of molecules, gases with higher molar masses will have higher densities.
Avogadro’s Law is a fundamental concept in the field of gases and has significant implications in various scientific and practical applications. It helps in determining the stoichiometry of chemical reactions, understanding the behavior of gases, and calculating gas volumes and densities.
Definition and statement of the law
Avogadro’s Law states that equal volumes of gases, at the same temperature and pressure, contain an equal number of particles, which can be atoms, molecules, or ions.
The statement of Avogadro’s Law can be summarized as follows: “Equal volumes of gases, under the same conditions of temperature and pressure, contain an equal number of particles.”
Key points about Avogadro’s Law:
- Equal Volumes: Avogadro’s Law focuses on the relationship between the volume of a gas and the number of particles it contains. It states that if two gases are at the same temperature and pressure, the volumes they occupy will have the same ratio as the number of particles present.
- Constant Temperature and Pressure: Avogadro’s Law holds true when the gases being compared are at the same temperature and pressure. This means that the law is applicable in ideal gas conditions, where temperature and pressure remain constant.
- Equal Number of Particles: Avogadro’s Law implies that gases have the same number of particles per unit volume at the same temperature and pressure. This means that gases with different molar masses will have different densities, but their volumes will contain an equal number of particles.
- Avogadro’s Number: Avogadro’s Law is directly related to Avogadro’s number (6.022 x 10^23), which represents the number of particles in one mole of any substance. Avogadro’s Law establishes that one mole of any gas, at the same temperature and pressure, will occupy the same volume as one mole of any other gas.
- Molar Volume: Avogadro’s Law allows for the determination of the molar volume of gases. The molar volume represents the volume occupied by one mole of a gas at a specific temperature and pressure. Under standard conditions (0 degrees Celsius and 1-atmosphere pressure), the molar volume is approximately 22.4 liters.
- Stoichiometry: Avogadro’s Law is essential in stoichiometric calculations, as it provides a relationship between the volume of a gas and the number of particles involved in a chemical reaction. This helps in determining the mole-to-volume ratios in reaction stoichiometry.
Avogadro’s Law is a fundamental concept in the field of gases and has significant implications in various scientific and practical applications. It is crucial in understanding the behavior of gases, determining molar volumes, and performing calculations involving gas volumes and particles.
Key Differences Between Gay-Lussac’s Law and Avogadro’s Law
While both Gay-Lussac’s Law and Avogadro’s Law are gas laws that describe the behavior of gases, they focus on different aspects and have distinct differences.
Here are the key differences between Gay-Lussac’s Law and Avogadro’s Law:
1. Relationship:
-
- Gay Lussac’s Law: It establishes a direct relationship between the pressure and temperature of a gas at a constant volume.
- Avogadro’s Law: It states that equal volumes of gases, at the same temperature and pressure, contain an equal number of particles (atoms, molecules, or ions).
2. Variables:
-
- Gay-Lussac’s Law: It relates pressure (P) and temperature (T), assuming constant volume.
- Avogadro’s Law: It relates volume (V) and the number of particles (n), assuming constant temperature and pressure.
3. Application:
-
- Gay-Lussac’s Law: It is crucial for understanding the effect of temperature changes on the pressure of a gas. It has applications in gas storage, transportation, and pressure regulation systems.
- Avogadro’s Law: It is used for determining the molar volume of gases, calculating stoichiometry in chemical reactions, and comparing the densities of gases.
4. Fundamental Quantity:
-
- Gay-Lussac’s Law: It is based on the relationship between pressure and temperature, two macroscopic properties of gases.
- Avogadro’s Law: It is based on the idea that equal volumes of gases contain the same number of particles, regardless of their nature or molar mass.
5. Combination with Other Laws:
-
- Gay-Lussac’s Law: It is often combined with other gas laws, such as Boyle’s Law or the ideal gas law, to describe the behavior of gases more comprehensively.
- Avogadro’s Law: It is often used in conjunction with other gas laws, such as the ideal gas law or the combined gas law, to perform calculations involving gas volumes and particles.
Gay-Lussac’s Law relates pressure and temperature at a constant volume, while Avogadro’s Law relates volume and the number of particles at constant temperature and pressure.
They have distinct applications and describe different aspects of gas behavior, making them valuable in different contexts within the field of gases.
Variables involved
The variables involved in Gay-Lussac’s Law and Avogadro’s Law are as follows:
Gay Lussac’s Law:
- Pressure (P): It represents the force exerted by a gas per unit area.
- Temperature (T): It measures the average kinetic energy of the gas molecules.
Avogadro’s Law:
- Volume (V): It represents the physical space occupied by gas.
- Number of particles (n): It refers to the number of atoms, molecules, or ions present in the gas.
It is important to note that in both laws, other variables are assumed to be constant to observe the relationship between the variables of interest.
In Gay-Lussac’s Law, the volume is assumed to remain constant, while in Avogadro’s Law, the temperature and pressure are assumed to remain constant.
Gay-Lussac’s Law vs Avogadro’s Law in Tabular Form
Sure! Here’s a comparison of Gay-Lussac’s Law and Avogadro’s Law in tabular form:
| Gay-Lussac’s Law | Avogadro’s Law | |
|---|---|---|
| Relationship | The direct relationship between pressure and temperature at constant volume | Direct relationship between volume and number of particles at constant temperature and pressure |
| Variables | Pressure (P) and temperature (T) | Volume (V) and number of particles (n) |
| Assumptions | Volume is constant | Temperature and pressure are constant |
| Application | Describes the effect of temperature changes on gas pressure | Determines molar volume, stoichiometry in chemical reactions, and comparison of gas densities |
| Equation | P₁/T₁ = P₂/T₂ | V₁/n₁ = V₂/n₂ |
| Molar Volume | Not directly related | Varies depending on the gas and conditions |
| Combination with Other Laws | Often combined with other gas laws, such as Boyle’s Law or the ideal gas law | Often used in conjunction with other gas laws, such as the ideal gas law or the combined gas law |
This tabular comparison highlights the key differences and characteristics of Gay-Lussac’s Law and Avogadro’s Law, including their relationships, variables involved, applications, and how they are combined with other gas laws.
Similarities and Overlapping Concepts
While Gay-Lussac’s Law and Avogadro’s Law have distinct differences, there are also some similarities and overlapping concepts between the two:
- Gas Behavior: Both laws are based on the behavior of gases and provide insights into the relationships between different gas properties.
- Ideal Gas Assumptions: Both laws assume ideal gas behavior, where gas molecules are considered to be point particles with no volume or intermolecular forces.
- Proportional Relationships: Both laws express a directly proportional relationship between two variables. Gay-Lussac’s Law relates pressure and temperature, while Avogadro’s Law relates volume and the number of particles.
- Temperature and Pressure: Although Avogadro’s Law does not directly involve temperature and pressure, it assumes constant temperature and pressure for the equality of volumes to hold. Gay-Lussac’s Law, on the other hand, explicitly examines the relationship between pressure and temperature.
- Combined Gas Law: Both laws can be incorporated into the combined gas law, which combines Boyle’s Law, Charles’s Law, Gay-Lussac’s Law, and Avogadro’s Law into a single equation. The combined gas law allows for simultaneous calculations involving changes in pressure, volume, temperature, and the number of particles.
- Stoichiometry: Both laws have implications in stoichiometric calculations and chemical reactions. Gay-Lussac’s Law is used to determine the effect of temperature on gas pressure, while Avogadro’s Law helps establish mole-to-volume ratios and stoichiometric relationships.
- Ideal Gas Law: The ideal gas law, which combines all the gas laws, including Gay-Lussac’s Law and Avogadro’s Law, is a comprehensive equation that relates the pressure, volume, temperature, and number of particles of a gas.
While Gay-Lussac’s Law and Avogadro’s Law have their distinct focuses, they are interconnected through their involvement in the overall understanding of gas behavior, stoichiometry, and the ideal gas law. They provide complementary insights into different aspects of gas properties and their relationships.
Conclusion
Gay-Lussac’s Law and Avogadro’s Law are two fundamental gas laws that describe different aspects of gas behavior. Gay-Lussac’s Law relates the pressure and temperature of a gas at constant volume, while Avogadro’s Law establishes that equal volumes of gases, at the same temperature and pressure, contain an equal number of particles.
Despite their differences, these laws also share similarities and overlapping concepts. Both laws assume ideal gas behavior, involve proportional relationships and have implications in stoichiometry and the combined gas law.
They contribute to our understanding of gas properties and are interconnected through the broader concept of the ideal gas law.
By studying and applying these laws, scientists and researchers gain insights into the behavior of gases, calculate gas volumes and densities, determine stoichiometry in chemical reactions, and ensure safe conditions in various industries involving gases.
Understanding the similarities, differences, and applications of Gay-Lussac’s Law and Avogadro’s Law helps us comprehend the complex behavior of gases and their interactions with temperature, pressure, volume, and the number of particles.