The key difference between HOMO and LUMO is that whereas HOMO is capable of donating electrons, LUMO is capable of accepting electrons.
The terms HOMO and LUMO fall under the sub-topic of “molecular orbital theory” in general chemistry. Where HOMO stands for “Highest Occupied Molecular Orbital”, There the term LUMO stands for “lowest occupied molecular orbital”.
So we call them “frontier orbitals”. A molecular orbital placed on any one atom gives the most probable position of the electron. Molecular orbitals are formed by combining the atomic orbitals of two separate atoms to separate their electrons.
These electrons are shared to form a covalent fastening between atoms. While forming these molecular orbitals, they separate into two forms HOMO and LUMO.
Importance of HOMO(Highest Occupied Molecular Orbital)
The highest occupied molecular orbital (HOMO) is of significant importance in molecular properties and chemical reactions. As the highest energy level among the occupied orbitals of a molecule, the HOMO plays an important role in many aspects of chemistry. One of its main functions is to donate electrons during chemical reactions.
Molecules with accessible and relatively low-energy HOMOs are more likely to donate electrons to other molecules or undergo nucleophilic reactions. This electron-donating ability makes the HOMO important in biosynthesis, catalysis, and the formation of new bonds.
The energy level of the HOMO determines the ionization energy of a molecule – the energy required to remove an electron. Thus, understanding the HOMO allows us to predict a molecule’s stability, reactivity, and susceptibility to oxidation processes.
Whether in drug discovery, physics, or biochemistry, realize the significance of HOMO. By providing invaluable insights into the behavior and interactions of molecules, driving innovation in scientific disciplines.
Importance of LUMO(Lowest Unoccupied Molecular Orbital)
The lowest unoccupied molecular orbital (LUMO) plays an important role in understanding molecular behavior and reactions. Orbital As the lowest energy level orbital among orbitals, the LUMO is the center of electron acceptance during chemical reactions.
Molecules with accessible and relatively high-energy LUMO levels are more likely to accept electrons from other molecules or participate in electrophilic reactions. This electron-accepting ability is fundamental for elucidating reaction mechanisms, designing catalysts, and predicting the behavior of molecules in various chemical processes.
The energy level of the LUMO is closely tied to the electron affinity of a molecule – the energy changes when an electron is added. This property affects the molecule’s response to electron-rich species. Thus, understanding the significance of the LUMO helps predict the reactions and interactions of molecules.
which has wide implications in areas such as materials science, environmental chemistry, and drug development. Through the lens of the LUMO, scientists gain essential insights into the electronic structure and potential reactions of molecules, facilitating innovative breakthroughs across scientific endeavors.
What is Homo?
HOMO stands for “Highest Occupied Molecular Orbital”. This is a concept of molecular orbital theory. which is used to describe the distribution of electrons in the orbitals of a molecule. Simply put, molecular orbitals are the regions of space where electrons are most abundant in a molecule.
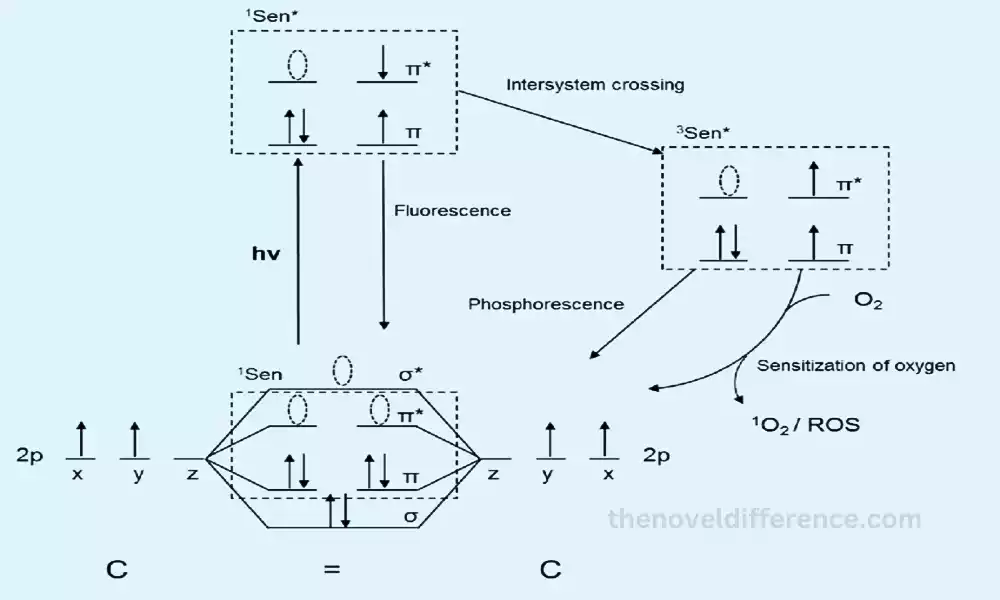
HOMO specifically refers to the molecular orbital with the highest energy level containing electrons. It is the orbital that is filled with electrons during the ground state configuration of the molecule. HOMO electrons play an important role in various chemical reactions.
especially those involving electron donation. Easily accessible and relatively low-energy molecules are more likely to donate electrons to other molecules. which makes it important in processes such as nucleophilic reactions and bond formation.
What is Lumo?
LUMO stands for “lowest unoccupied molecular orbital.” Similar to HOMO (Highest Occupied Molecular Orbital), LUMO is a concept in molecular orbital theory that deals with the distribution of electrons in molecular orbitals.
LUMO refers to the molecular orbital with the lowest energy level. which is currently unoccupied by electrons in the ground state configuration of a molecule. It is the orbital that is available to accept extra electrons during chemical reactions.
Electrons in the LUMO are involved in processes that require electron acceptance, such as electrophilic reactions and reactions with electron-rich species.
Difference Between HOMO and LUMO
| Aspect | HOMO | LUMO |
|---|---|---|
| Position in Energy Diagram | Highest-energy occupied molecular orbital | Lowest-energy unoccupied molecular orbital |
| Electron Occupation | Contains electrons | Does not contain electrons |
| Electron Distribution | Highest-energy electrons are loosely bound and more reactive | Electrons are absent, making the orbital electron-deficient |
| Reactivity Role | Donates electrons, nucleophilic reactions | Accepts electrons, electrophilic reactions |
| Ionization Energy | The energy required to remove an electron correlates with HOMO energy | Related to the energy difference between LUMO and vacuum level |
| Stability Influence | A larger HOMO-LUMO energy gap indicates greater stability | A smaller energy gap can lead to greater reactivity |
| Charge Distribution | Contributes to charge distribution in chemical reactions | Participates in charge transfer and bond formation |
| Lewis Base Behavior | Donates electron pairs, acts as a Lewis base | Receives electron pairs, acts as a Lewis acid |
| Role in Redox Reactions | Provides electrons for reduction reactions | Accepts electrons in oxidation reactions |
| Reaction Kinetics | Influences reaction rates through electron donation | Influences rates by electron acceptance |
| Photochemical Reactions | Participates in transitions during photochemical processes | Excitation of electrons from HOMO to LUMO initiates reactions |
| Material Design (Electron Donors) | Important in designing conductive and electron-donating materials | Important in designing electron-accepting materials |
| Electron Density | Contains electron density | Absence of electron density |
| Interaction with Other Orbitals | Can interact with LUMOs of other molecules to form new bonds | Can interact with HOMOs of other molecules to facilitate reactions |
Remember that the properties and behaviors of HOMO and LUMO are interconnected, and their roles are complementary in understanding molecular electronic structure, chemical reactivity, and a wide range of chemical and physical phenomena.
Role in electron donation and chemical reactivity
The Highest Occupied Molecular Orbital (HOMO) plays a crucial role in electron donation and chemical reactivity. Its energy level and electron distribution influence a molecule’s ability to participate in various types of chemical reactions.
Here’s how the HOMO contributes to electron donation and chemical reactivity:
- Electron Density and Accessibility: The HOMO is the molecular orbital that holds the highest-energy electrons in a molecule. These electrons are generally more loosely bound and are therefore more likely to be involved in chemical interactions. Molecules with easily accessible HOMOs are prone to donating electrons to other molecules.
- Nucleophilic Reactions: Nucleophilic reactions involve the attack of an electron-rich species (nucleophile) on an electron-poor species (electrophile). Molecules with available electrons in their HOMOs can act as nucleophiles, initiating reactions by donating electrons to electrophiles. This is a fundamental mechanism in many organic, inorganic, and biochemical reactions.
- Lewis Base Behavior: A Lewis base is a molecule that donates an electron pair. Molecules with high electron density in their HOMOs have a greater tendency to behave as Lewis bases by donating electron pairs to Lewis acids (electron acceptors).
- Formation of Covalent Bonds: Covalent bond formation involves the sharing of electron pairs between atoms. Molecules with accessible HOMOs can contribute their electrons to form covalent bonds with other atoms or molecules, leading to the creation of new compounds.
- Reaction Kinetics: The rate at which a reaction occurs can be influenced by the presence of accessible electrons in the HOMO. Reactions involving molecules with higher HOMO electron density tend to proceed more rapidly due to the increased probability of electron transfer.
- Electron Transfer Reactions: Electron transfer reactions involve the movement of electrons from one molecule to another. Molecules with available electrons in their HOMOs can act as electron donors, facilitating electron transfer processes in chemical reactions and electrochemical systems.
- Radical Reactions: Radicals are highly reactive species with unpaired electrons. Molecules with electrons in their HOMOs can readily undergo radical reactions by donating or accepting unpaired electrons, leading to the formation of new bonds and products.
- Redox Reactions: Redox reactions involve the transfer of electrons between reactants. Molecules with electrons in their HOMOs can participate in redox reactions by donating electrons to reduce other species while being oxidized themselves.
- Electrophilic Substitution: While the HOMO is more commonly associated with nucleophilic reactions, it can also influence electrophilic substitution reactions. The HOMO can interact with electrophiles by donating electron density to the electrophilic center, facilitating bond formation.
The HOMO’s role in electron donation and chemical reactivity is pivotal for understanding how molecules interact, transform, and react with one another. The accessibility of electrons in the HOMO determines a molecule’s capacity to donate electrons, initiate reactions, and participate in a wide range of chemical processes.
Connection to molecular reactivity and electron affinity
The Lowest Unoccupied Molecular Orbital (LUMO) has a direct connection to molecular reactivity and electron affinity, both of which play pivotal roles in understanding a molecule’s behavior and chemical interactions.
Here’s how the LUMO is linked to these concepts:
- Molecular Reactivity:
-
- Electron Acceptance: The LUMO’s energy level determines a molecule’s ability to accept electrons. Molecules with accessible LUMOs have lower energy thresholds for accepting electrons, making them more susceptible to electrophilic reactions, where electron-deficient species (electrophiles) react with electron-rich species.
- Reaction Mechanisms: The interaction between the LUMO of one molecule and the Highest Occupied Molecular Orbital (HOMO) of another molecule plays a central role. This interaction facilitates the transfer of electrons, the formation of new bonds, and the rearrangement of atoms during a reaction.
- Electron Affinity:
-
- Definition: Electron affinity refers to the energy change that occurs when a neutral atom or molecule gains an electron to form a negatively charged ion. The LUMO energy level is directly related to the electron affinity, as it represents the energy required to promote an electron from the LUMO to the vacuum level (free electron state).
- Electron Accepting Tendency: Molecules with lower LUMO energy levels have a greater electron affinity, indicating a stronger tendency to accept electrons and form negatively charged ions. This property is crucial in various chemical and physical processes, such as in redox reactions and charge transfer phenomena.
- Lewis Acid Behavior:
-
- LUMO and Lewis Acids: The LUMO’s energy level influences a molecule’s behavior as a Lewis acid, which is a substance that can accept an electron pair. Molecules with low-energy LUMOs tend to act as Lewis acids by accepting electron pairs from Lewis bases, promoting bond formation.
- Reaction Kinetics:
-
- LUMO and Reaction Rates: The LUMO of the electron-accepting species determines the energy barrier for the electron transfer process. Molecules with lower LUMO energy levels enable faster electron transfer, leading to higher reaction rates.
- Photochemical Reactions:
-
- Excitation and LUMO: Excitation of an electron from the HOMO to the LUMO can lead to the initiation of chemical transformations. The energy required for this excitation corresponds to the energy difference between the HOMO and LUMO energy levels.
- Redox Reactions:
-
- LUMO and Oxidation: The LUMO energy level of a molecule influences its ability to accept electrons during redox reactions. Molecules with accessible LUMOs are more likely to undergo oxidation by accepting electrons from reducing agents.
The LUMO energy level is a key determinant of a molecule’s reactivity, its ability to accept electrons and its affinity for electrons. It influences the likelihood of electron transfer, bond formation, and other chemical interactions. Understanding the LUMO’s role provides insights into a wide range of chemical processes and helps in predicting a molecule’s behavior in various contexts.
Conclusion
It is most important to understand the difference between HOMO and LUMO. These energy levels serve as the basis for understanding chemical reactions. which provides insight into the multitude of reactions that shape the world in chemistry.
From organic transformations to photochemical processes, HOMO and LUMO play important roles, enriching our understanding of the molecular universe.
