Various reactions occur to transform molecules from one form to another. Among these reactions, oxidative addition and reductive elimination play significant roles. These processes involve the breaking and formation of chemical bonds, ultimately leading to the creation of new compounds. In this article, we will delve into the difference between oxidative addition and reductive elimination, exploring their mechanisms, applications, and implications in various chemical reactions.
Importance of oxidative addition and reductive elimination reactions
Oxidative addition and reductive elimination reactions play crucial roles in transition metal catalysis, making them of significant importance in various chemical processes. These reactions are fundamental steps in the activation and transformation of substrates, enabling the formation of new bonds and the regeneration of the metal center.
Oxidative addition involves the coordination of a substrate to a metal center, followed by the activation of a substrate bond and the formation of new metal-substrate bonds. This process allows for the insertion of the metal into otherwise unreactive bonds, such as carbon-heteroatom or metal-hydrogen bonds.
By facilitating these bond activations, oxidative addition enables the generation of reactive intermediates and the initiation of subsequent catalytic cycles. Reductive elimination serves as the reverse process of oxidative addition. It involves the activation of metal-substrate bonds, the migration of the substrate to a coordinated position, and the subsequent formation of new substrate bonds. Reductive elimination is responsible for the formation of new bonds, such as carbon-carbon, carbon-heteroatom, or metal-hydrogen bonds, and it plays a critical role in the final step of many catalytic cycles.
Understanding oxidative addition and reductive elimination reactions is essential for designing and optimizing transition metal catalysis. These reactions dictate the scope and applicability of catalytic processes, as different substrates exhibit varying reactivity towards these transformations. Moreover, the conditions required for efficient oxidative addition and reductive elimination reactions can significantly influence the overall efficiency of a catalytic system.
Oxidative addition and reductive elimination reactions have implications for stereochemistry and regiochemistry in catalysis. The stereochemical outcome of these reactions can lead to the formation of chiral products, enabling the synthesis of enantiomerically enriched compounds. Regioselectivity, Determines the preferred site of bond activation or formation, which is crucial for achieving desired product selectivity in catalytic transformations.
The rate-determining steps in oxidative addition and reductive elimination reactions can significantly impact the overall rate of a catalytic process. Understanding the factors that govern the kinetics of these steps allows for the optimization of catalytic conditions and the development of more efficient catalytic systems.
Oxidative addition and reductive elimination reactions are fundamental processes in transition metal catalysis. They enable the activation and transformation of substrates, control the stereochemistry and regiochemistry of reactions, and influence the kinetics and efficiency of catalytic processes. Advances in understanding and harnessing these reactions have opened up new avenues for synthetic chemistry, enabling the development of more sustainable and selective chemical transformations.
Definition of Oxidative Addition and Reductive
Oxidative Addition: Oxidative addition is a reaction in transition metal chemistry where a substrate molecule coordinates with a transition metal center, resulting in the formation of new metal-substrate bonds. This reaction involves the transfer of electron density from the substrate to the metal, leading to the oxidation of the metal center. The oxidative addition process often involves the activation of relatively inert bonds, such as carbon-heteroatom or metal-hydrogen bonds, allowing for subsequent transformations and the generation of reactive intermediates.
Reductive Elimination: Reductive elimination is the reverse process of oxidative addition in transition metal chemistry. It involves the cleavage of metal-substrate bonds, resulting in the formation of new substrate molecules and the regeneration of the metal center. This reaction typically occurs as the final step in a catalytic cycle, where a coordinated substrate migrates to a position adjacent to the metal center and undergoes bond cleavage. Reductive elimination often leads to the formation of new carbon-carbon, carbon-heteroatom, or metal-hydrogen bonds, and it plays a crucial role in the overall efficiency and selectivity of catalytic processes.
What is Oxidative Addition?
Oxidative addition is a fundamental reaction in transition metal chemistry, where a substrate molecule coordinates with a transition metal center, resulting in the formation of new metal-substrate bonds. In this process, the metal center undergoes oxidation by gaining electron density from the substrate. Typically, oxidative addition involves the activation of relatively inert bonds, such as carbon-heteroatom or metal-hydrogen bonds, which are often unreactive under standard conditions.
By coordinating with the metal center, the substrate bond becomes more susceptible to further transformations and enables the generation of reactive intermediates. Oxidative addition reactions are widely employed in various catalytic processes, allowing for the insertion of transition metals into organic and inorganic substrates and facilitating subsequent bond-forming reactions.
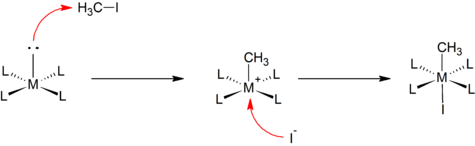
Reaction mechanism
The reaction mechanism of oxidative addition involves several steps that collectively lead to the formation of new metal-substrate bonds. While the specific details can vary depending on the reaction and the nature of the substrate and metal complex involved.
The general mechanism can be described as follows:
1. Coordination: The first step in oxidative addition is the coordination of the substrate molecule to the metal center. This occurs through the interaction of a lone pair of electrons on the substrate with an empty orbital on the metal. The coordination can take place via sigma (σ) or pi (π) bonding interactions, depending on the nature of the substrate and the metal complex.
2. Activation of the substrate bond: Once coordinated with the metal, the substrate bond undergoes activation. This activation can involve bond elongation, bond polarization, or bond cleavage, depending on the specific reaction. The metal center facilitates the redistribution of electron density within the substrate, making it more reactive.
3. Insertion: Following the activation of the substrate bond, the metal center inserts itself into the bond. This results in the formation of new metal-substrate bonds. The metal atom becomes directly bonded to the substrate, often leading to the generation of a complex with altered coordination and oxidation states.
4. Rearrangement and ligand exchange: The coordination geometry around the metal center may undergo rearrangement to achieve a more stable configuration. Ligand exchange reactions may also occur, where existing ligands on the metal complex are displaced by the newly formed metal-substrate bonds.
Oxidative addition reactions involve the coordination, activation, insertion, and potential rearrangement of substrate molecules by transition metal complexes. These steps collectively enable the formation of new metal-substrate bonds and initiate subsequent transformations, making oxidative addition a key process in transition metal catalysis.
Activation of the substrate bond
The activation of the substrate bond in oxidative addition is a critical step that prepares the bond for subsequent reactions and facilitates the formation of new metal-substrate bonds. The specific nature of the activation process depends on the type of substrate and the reaction conditions.
There are several common mechanisms involved:
1. Bond elongation: The substrate bond is stretched or elongated upon coordination with the metal center. This stretching weakens the bond and makes it more susceptible to further transformations. The metal complex provides a favorable environment for the elongation of the bond by stabilizing the transition state.
2. Bond polarization: The metal center can induce polarization of the substrate bond by withdrawing electron density from or donating electron density to the bond. This electronic redistribution leads to an uneven distribution of charge along the bond, making it more reactive. Polarization can result from the electronegativity difference between the metal and the substrate or the presence of electron-donating or electron-withdrawing ligands on the metal complex.
3. Bond cleavage: The substrate bond undergoes direct cleavage upon coordination with the metal center. This cleavage can occur homolytically or heterolytically, depending on the nature of the bond and the reaction conditions. Homolytic cleavage involves the equal division of electrons between the two fragments, while heterolytic cleavage leads to the formation of charged species with opposite charges on each fragment.
The activation of the substrate bond is driven by the interaction between the substrate and the metal center. The metal’s electronic and steric properties, as well as the nature of the ligands surrounding the metal, play crucial roles in promoting bond activation. By destabilizing the substrate bond and redistributing electron density, the metal complex facilitates subsequent reactions, such as bond insertion and ligand exchange, leading to the formation of new metal-substrate bonds.
It is important to note that the specific mechanisms and factors involved in substrate bond activation can vary depending on the reaction and the metal complex employed. Different transition metals and ligand environments exhibit diverse reactivity patterns, allowing for a wide range of oxidative addition reactions to occur.
What is Reductive Elimination?
Reductive elimination is a fundamental reaction in transition metal chemistry that involves the cleavage of a metal-substrate bond, leading to the formation of new substrate molecules and the regeneration of the metal center. The metal center undergoes reduction by transferring electron density to the substrate, resulting in the formation of new bonds between the substrate fragments.
The general mechanism of reductive elimination can be described as follows:
1. Activation of metal-substrate bond: The first step in reductive elimination is the activation of the metal-substrate bond. This activation can be achieved by various factors, including changes in the metal’s oxidation state, coordination geometry, or the influence of external factors such as temperature or ligand environment. Activation of the metal-substrate bond weakens the bond, making it more susceptible to cleavage.
2. Migration of the substrate: Once the metal-substrate bond is activated, the substrate fragment migrates to a position adjacent to the metal center. This migration can occur via intramolecular or intermolecular processes, depending on the reaction conditions and the nature of the substrate and metal complex.
3. Bond cleavage: Following the migration of the substrate, the metal-substrate bond undergoes cleavage. This cleavage can occur homolytically or heterolytically. Homolytic cleavage involves the equal distribution of electrons between the two fragments, while heterolytic cleavage leads to the formation of charged species with opposite charges on each fragment.
4. Formation of new bonds: After bond cleavage, new bonds are formed between the substrate fragments. These bonds can involve carbon-carbon, carbon-heteroatom, or metal-hydrogen bond formation, depending on the specific reaction and the nature of the substrate and metal complex. The formation of new bonds between the substrate fragments completes the reductive elimination process.
Reductive elimination is often the final step in a catalytic cycle, leading to the release of the desired product and the regeneration of the active metal catalyst. The efficiency and selectivity of reductive elimination are influenced by factors such as the nature of the metal complex, the substrate, the ligand environment, and reaction conditions.

Reductive elimination plays a crucial role in transition metal catalysis by enabling the formation of new bonds and facilitating the controlled release of products. Understanding the mechanisms and factors governing reductive elimination is essential for designing and optimizing catalytic processes.
Reaction mechanism
The reaction mechanism of reductive elimination involves several steps that collectively result in the cleavage of a metal-substrate bond and the formation of new substrate molecules. While the specific details can vary depending on the reaction and the nature of the substrate and metal complex involved.
The general mechanism can be described as follows:
1. Activation of the metal-substrate bond: The first step in reductive elimination is the activation of the metal-substrate bond. This activation can occur through changes in the metal’s oxidation state, coordination geometry, or through the influence of external factors such as temperature or ligand environment. The activation weakens the bond, making it more susceptible to cleavage.
2. Migration of the substrate: Once the metal-substrate bond is activated, the substrate fragment migrates to a position adjacent to the metal center. This migration can involve intramolecular rearrangement within the metal complex or intermolecular processes, where the substrate interacts with another molecule or ligand bound to the metal.
3. Cleavage of the metal-substrate bond: Following substrate migration, the metal-substrate bond undergoes cleavage. This cleavage can occur through homolytic or heterolytic processes. The bond breaks symmetrically, with each fragment receiving one of the electrons from the bond. Heterolytic cleavage involves an uneven distribution of electrons, resulting in the formation of charged species with opposite charges on each fragment.
4. Formation of new bonds: After the cleavage of the metal-substrate bond, new bonds are formed between the substrate fragments. These new bonds can involve the formation of carbon-carbon, carbon-heteroatom, or metal-hydrogen bonds, depending on the specific reaction and the nature of the substrate and metal complex.
The overall reductive elimination process leads to the release of the desired product and the regeneration of the active metal catalyst, which can subsequently participate in further catalytic cycles.
It is important to note that the specific mechanisms and factors involved in reductive elimination can vary depending on the reaction and the metal complex employed. Different transition metals and ligand environments exhibit diverse reactivity patterns, allowing for a wide range of reductive elimination reactions to occur. Additionally, the stereochemistry and regiochemistry of the reaction can be influenced by the geometry of the metal complex and the spatial arrangement of the substrate fragments.
Migration of substrate to a coordinated position
The migration of the substrate to a coordinated position refers to the movement or rearrangement of the substrate fragment within the metal complex, positioning it near the metal center. This step is a crucial part of the reductive elimination process and occurs after the activation of the metal-substrate bond.
The migration of the substrate can occur through intramolecular or intermolecular processes, depending on the specific reaction and the nature of the substrate and metal complex.
Intramolecular migration: The substrate fragment undergoes a rearrangement within the metal complex itself. This rearrangement often involves the rotation or movement of a specific group or moiety within the substrate, bringing it closer to the metal center. The intramolecular migration can be facilitated by the flexibility and coordination geometry of the metal complex, as well as the presence of appropriate ligands that can stabilize the transition state.
Intermolecular migration: The substrate interacts with another molecule or ligand bound to the metal center. This can occur through coordination to a vacant site on the metal complex or through transient bonding interactions with nearby ligands. Intermolecular migration can involve the transfer of the substrate fragment from one molecule to another or the exchange of ligands between the substrate and a coordinating species.
The migration of the substrate to a coordinated position is necessary for the subsequent cleavage of the metal-substrate bond and the formation of new bonds. Positioning the substrate fragment near the metal center increases the likelihood of bond cleavage occurring at the desired location and facilitates the formation of new bonds with adjacent atoms or ligands.
The specific mechanism and factors governing the migration of the substrate depend on the reaction conditions, the nature of the metal complex, and the characteristics of the substrate molecule. The geometry, steric effects, and electronic properties of the metal complex, as well as the nature of the coordinating ligands and the substrate itself, all influence the migration process.
Difference between Oxidative Addition and Reductive Elimination
Oxidative addition and reductive elimination are two fundamental processes in transition metal chemistry that occur in catalytic cycles. While they are interconnected and often occur sequentially, there are significant differences between these two reactions.
Here are the key distinctions:
1. Concept:
• Oxidative Addition: Oxidative addition involves the coordination of a substrate molecule to a metal center, leading to the formation of new metal-substrate bonds.
• Reductive Elimination: Reductive elimination, on the other hand, is the reverse process of oxidative addition. It involves the cleavage of a metal-substrate bond, resulting in the formation of new substrate molecules and the regeneration of the metal center.
2. Bond Activation:
• Oxidative Addition: The substrate bond is activated, typically through bond elongation, bond polarization, or bond cleavage, making it more susceptible to reaction with the metal center.
• Reductive Elimination: The metal-substrate bond is cleaved, resulting in the formation of new bonds between the substrate fragments.
3. Electron Transfer:
• Oxidative Addition: Oxidative addition involves the transfer of electron density from the substrate to the metal center, leading to the oxidation of the metal.
• Reductive Elimination: Reductive elimination involves the transfer of electron density from the metal center to the substrate, resulting in the reduction of the metal.
4. Timing:
• Oxidative Addition: Oxidative addition usually occurs as the initial step in a catalytic cycle, initiating the transformation and generating reactive intermediates.
• Reductive Elimination: Reductive elimination typically occurs as the final step in a catalytic cycle, releasing the desired product and regenerating the active metal catalyst.
5. Bond Formation:
• Oxidative Addition: Oxidative addition leads to the formation of new metal-substrate bonds, allowing for subsequent transformations.
• Reductive Elimination: Reductive elimination leads to the formation of new bonds between the substrate fragments, resulting in the release of the desired product.
6. Driving Forces:
• Oxidative Addition: Oxidative addition is driven by the reduction of the metal center and the stabilization of the resulting metal-substrate complex.
• Reductive Elimination: Reductive elimination is driven by the formation of stable products and the regeneration of the active metal catalyst.
Oxidative addition involves the activation and coordination of the substrate to the metal center, leading to the formation of new metal-substrate bonds. Reductive elimination, on the other hand, involves the cleavage of a metal-substrate bond, resulting in the formation of new substrate molecules. These reactions are interconnected and play crucial roles in transition metal catalysis, enabling the activation and transformation of substrates in a controlled manner.
Comparison Chart
Sure! Here’s a comparison chart highlighting the key differences between oxidative addition and reductive elimination:
| Oxidative Addition | Reductive Elimination | |
|---|---|---|
| Concept | Formation of new metal-substrate bonds | Cleavage of metal-substrate bond |
| Bond Activation | Activation of substrate bond | Cleavage of metal-substrate bond |
| Electron Transfer | Electron transfer from the substrate to the metal | Electron transfer from metal to the substrate |
| Timing | The initial step in a catalytic cycle | The final step in a catalytic cycle |
| Bond Formation | New metal-substrate bonds formed | New bonds formed between substrate fragments |
| Driving Forces | Metal center reduction, stabilization of metal-substrate complex | Formation of stable products, regeneration of the metal catalyst |
| Role in Catalysis | Initiates the catalytic cycle, generates reactive intermediates | Releases desired product regenerates the active catalyst |
| Importance | Activates inert bonds enable subsequent transformations | Controls selectivity, the efficiency of catalytic processes |
This chart summarizes the main distinctions between oxidative addition and reductive elimination, including their concepts, bond activation/cleavage, electron transfer direction, timing within catalytic cycles, bond formation, driving forces, role in catalysis, and their overall importance in transition metal chemistry.
Conclusion
Oxidative addition and reductive elimination are distinct processes that occur in various chemical reactions. Oxidative addition involves the formation of a new bond and an increase in the metal’s oxidation state, while reductive elimination leads to a decrease in the metal’s oxidation state and the formation of a new bond.
Understanding these mechanisms and their applications paves the way for the development of new catalysts, the synthesis of complex molecules, and advancements in medicinal chemistry. By harnessing the power of oxidative addition and reductive elimination, scientists continue to unlock new possibilities in the world of chemistry.
